Nanofiltration Membrane
Water Treatment System & Membrane Selection and Ordering Can Be Easy & Safe
Whether you are replacing an old project or designing a new project, we can provide it for you based on our rich experience. We have professional sales staff to connect with you to solve all your problems from purchasing to using the MBR membrane.Nanofiltration membrane parameters
| Model | Average Permeate GPD(m3/d) | Active Membrane ft2(m2) | NaCl Rejection % | CuSO4 Rejection % | |
| 30 Series | NF30-2521 | 360 (1.4) | 12 (1.1) | 30~40 | 96 |
| NF30-2540 | 780 (3) | 28 (2.6) | |||
| NF30-4021 | 1000 (3.8) | 33 (3.1) | |||
| NF30-4040 | 2400 (9.1) | 90 (8.4) | |||
| NF30-8040 | 11000(41.6) | 400(37.2) | |||
| 90 Series | NF90-2521 | 340 (1.3) | 12 (1.1) | 80~90 | 98 |
| NF90-2540 | 700 (2.6) | 28 (2.6) | |||
| NF90-4021 | 900 (3.4) | 33 (3.1) | |||
| NF90-4040 | 2200 (8.3) | 90 (8.4) | |||
| NF90-8040 | 10500 (39.7) | 400 (37.2) | |||
Test conditions
| Testing Pressure | 70 psi (0.48MPa) |
| Temperature of Testing Solution | 25 ℃ |
| Concentration of Testing Solution (NaCl) | 500ppm NaCl; 2000ppm MgS04 |
| pH Value of Testing Solution | 8 |
| Recovery Rate of Single Element | 15% |
Operation Limits & Conditions
| Max. Working Pressure | 600psi (4.14Mpa) |
| Max. Feed Water Flow | 8040 75 gpm (17 m3/h) |
| 4040 16 gpm (3.6 m3/h) | |
| 2540 6 gpm (1.4 m3/h) | |
| Max. Feed Water Temperature | 45 0C |
| Max. Feed Water SDl15 | 5 |
| Residual Chlorine Concentration of FeedWater | <0.1 mg/L |
| Max.Pressure Drop of Single Element | 15psi (0.1Mpa) |
| PH Range | 3-10 |
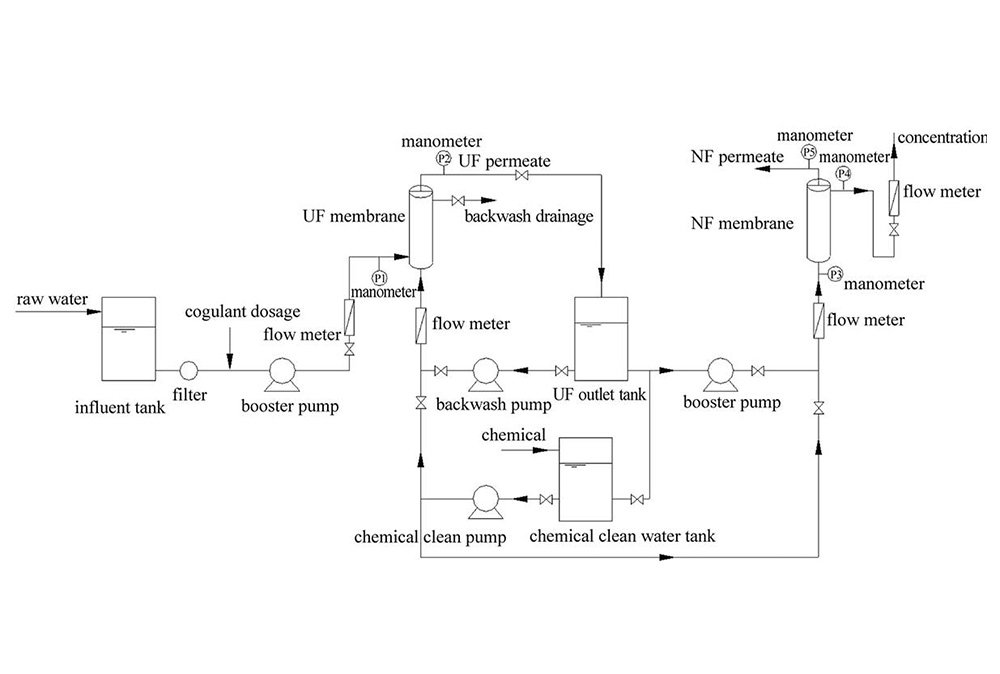
System Design
Support system customized design, please tell us your needs and we will provide you with the best solution
Production Lines and Warehouses






Application Areas

- Seawater Desalination

- Oil and water separation

- liquid concentration
- Remove microorganisms

- Remove color

- Reduce water hardness
What is the difference between RO/NF?
Although RO and NF are very similar, they can be distinguished based on the size of particles each is capable of removing. In contrast, RO and NF are able to remove finer contaminants than MF and UF, and their applications include removal of hardness, nitrates, sulfates, total dissolved solids (TDS), heavy metals, radionuclides from processes and waste streams and organic macromolecules.Reverse Osmosis
RO is the best of all membrane filtration systems, with extremely small pore sizes capable of removing particles as small as 0.1 nm. RO has been around since the 1950s and is used primarily for desalination, such as producing drinking water from seawater or brackish water sources. Other applications for RO include filtration of process water in industrial applications such as the printing industry to maintain optimal equipment performance. RO membranes are very effective at removing all ions, regardless of size.Nanofiltration
NF has a slightly coarser filtration effect than RO, capable of removing particles as small as 0.002 to 0.005 μm in diameter. Nanofiltration is a relatively new technology developed primarily for drinking water production. Nanofiltration removes harmful contaminants, such as pesticide compounds and organic macromolecules, while retaining minerals that reverse osmosis would remove. Nanofiltration membranes remove larger divalent ions (such as calcium sulfate) while allowing the passage of smaller monovalent ions (such as sodium chloride).What is the filtration principle of nanofiltration membrane?
Nanofiltration membrane is a new type of separation membrane that came out in the late 1980s. The pore size of the nanofiltration membrane is above 1nm, and the general specification is 1-2nm. The molecular weight cutoff of the nanofiltration membrane is between the reverse osmosis membrane and the ultrafiltration membrane. The topic that Blue Membrane will talk about today is related to the molecular weight cutoff of nanofiltration membranes, that is, why the rejection rate of nanofiltration membranes for divalent ions is higher than the rejection rate for monovalent ions. The reason why the rejection rate of nanofiltration membranes for divalent ions is higher than that for monovalent ions In fact, the reason why the rejection rate of divalent ions by nanofiltration membranes is higher than that of monovalent ions is ultimately due to the Dornan effect of nanofiltration membranes. That's right, it's the Dornan effect, one of the mechanisms by which nanofiltration membranes intercept ions. Because most nanofiltration membranes are composite membranes, the separation layer on the surface of the nanofiltration membrane is composed of polyelectrolytes, so it has a certain rejection rate for inorganic salts. The molecular weight cutoff range of the nanofiltration membrane is approximately: 100-2000Da The reason why the rejection rate of nanofiltration membranes for divalent ions is higher than that of monovalent ions: Introduction to the Dornan effect According to Baidu Encyclopedia's introduction to the Dornan effect: When a charged base film is placed in a salt-containing solvent, the counterions in the solution (ions with charges opposite to the fixed charge) are in the film. The concentration of ions is greater than its concentration in the main liquid, while in the main liquid, the concentration of ions with the same name in the film is lower than its concentration in the main liquid. Therefore, the generated Donnan potential prevents the diffusion of ions of the same name from the bulk solution into the membrane. In order to maintain electrical neutrality, counter ions can also be trapped by the membrane. The charged groups in most charged reverse osmosis membranes are sulfonic acid groups and carboxylic acid groups with negative electrons. The film rejection rate (R) is roughly as follows: R=1-K This model relates the film rejection rate to the charge capacity, the solute concentration in the material solution, and the charge number of the ions, but does not consider the effects of diffusion and convection in the charged film, which are equally important in the charged film. Conclusion: Therefore, a conclusion can be drawn from the above principle: the rejection rate of nanofiltration membranes for soluble monovalent ions is lower than that of high-valent ions. Therefore, it can explain why the rejection rate of nanofiltration membranes for divalent ions is lower than that of monovalent ions. High ion rejection rate Experimental conclusions on the rejection rates of nanofiltration membranes for different ions and different valence states: Researchers have previously conducted studies on different ion rejection rates. (1) The valence state of ions affects the rejection rate of inorganic salts. However, the valence state of anions has a small impact on the rejection rate, while the valence state of cations has a greater impact on the rejection rate. The rejection rate of nanofiltration membranes for divalent (and trivalent) cations is higher than that for monovalent cations. Rate. Many ions exist in divalent or high-valent forms in water (such as Mg2+, Ni2+, Cu2+, Mn2+, Cd2+, Fe2+, etc.). They can be effectively removed from the water body by using the separation characteristics of nanofiltration membranes. (2) The nanofiltration membrane has a higher rejection rate of complex ions. (3) The ion rejection rate has a certain relationship with the Shannon radius. The anion rejection rate has the same change pattern as the Shannon radius, while the cation rejection rate has the opposite change pattern as the Shannon radius. If you think this information is good, in the next issue, Blue Membrane Water Treatment will compile a specific experimental process of the rejection rate of different ions and different valence states by nanofiltration membranes.Exthbition
Shanghai CM's 2023 global exhibition live events
Manila, Philippines Mar 22-24, 2023
Jakarta, Indonesia Aug 31-Sep 1, 2023

Moscow, Russia Sep 12-14, 2023

Dubai, United Arab Emirates Nov 15-17, 2023









